GemVax Announces Topline Results from Phase 2a Progressive Supranuclear Palsy Clinical Trial at Neuro2024
페이지 정보
작성자 관리자 조회4,417 views 작성일 24-10-29 15:08본문
GemVax Announces Topline Results from Phase 2a Progressive Supranuclear Palsy Clinical Trial at Neuro2024
Topline supports moving to Phase 3 trial and shows
potential to develop GV1001as the world’s first PSP treatment
GemVax & KAEL Co., Ltd. (“GemVax”; KOSDAQ
ticker: 082270) announced that topline results of a Phase 2a clinical trial (the
“Phase 2a PSP Clinical Trial”) of GV1001, an investigational peptide drug for
the treatment of progressive supranuclear palsy (“PSP”), were presented at “Neuro2024:
The PSP and CBD International Research Symposium” in Toronto, Canada, at 4:45
p.m. local time on 24th October.
PSP is a degenerative disease that, like
Parkinson’s disease, causes symptoms such as gait disturbances, early falls,
vertical gaze palsy, rigidity, tremors, and cognitive decline, but it
progresses faster and currently has no fundamental treatment. PSP is classified
into several types, including PSP-Richardson's syndrome (“PSP-RS”) and
PSP-parkinsonism (“PSP-P”). Compared to other types of PSP, the PSP-RS type
shows a greater accumulation of tau protein and affects larger areas, including
the cerebellum, dentate nucleus, pontine nuclei, frontal lobe, and parietal
lobe.
The Phase 2a PSP Clinical Trial was a 24-week,
randomized, double-blind, placebo-controlled, prospective exploratory clinical
trial conducted in 78 patients with PSP at 5 centers in Korea. The participants
were randomized 1:1:1 to receive either placebo or GV1001 0.56 mg or GV1001
1.12 mg administered subcutaneously once weekly for the first 4 weeks (1
month), and then at 2-week intervals for 20 weeks (5 months) for a total of 24
weeks (6 months). Patients with both PSP-RS and PSP-P types were eligible to
participate in the study. Results showed higher benefits in the lower dose
group (0.56 mg), particularly in PSP-RS type patients.
The primary endpoint of the trial was change from baseline in total score (calculated as the least-square mean using MMRM method) of PSP-Rating Scale after 24 weeks of GV1001 administration, which showed deterioration by 2.14 points in GV1001 0.56 mg dose group compared to 4.10 points in the placebo group, demonstrating a 48% reduction in disease progression (see Figure 1). Although statistical significance was not demonstrated, the results support the potential of GV1001 as a treatment of PSP, a disease for which there is currently no cure, and the potential to advance GV1001 into further clinical trials.
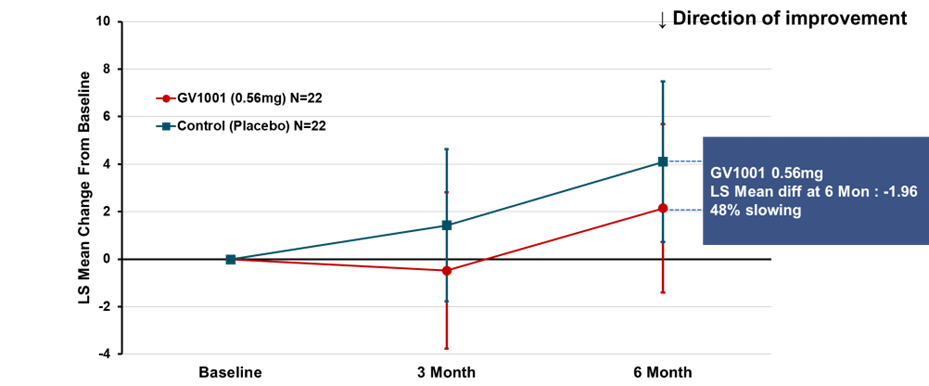
Figure 1. PSP-Rating
Scale Total Score (PSP-RS Type + PSP-P Type; LS mean using MMRM)
The clinically typical PSP is often referred to
as the PSP-RS type, which accounts for the majority of PSP patients. This type
progresses faster and has a shorter average survival time compared to other PSP
types. Subgroup analysis was conducted in patients with PSP-RS type only. The
change from baseline in PSP-Rating Scale total score mean (calculated using
simple average) at 24 weeks of GV1001 administration to PSP-RS type patients was
a deterioration by 0.25 points in the GV1001 0.56 mg dose group compared to a
deterioration by 5.19 points in the placebo group, demonstrating a 4.94-point
difference or a 95% reduction in disease progression (see Figure 2).
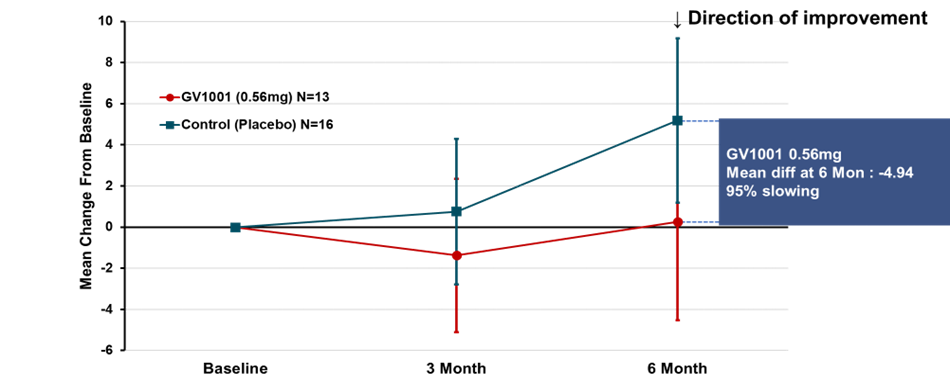
Many PSP-RS type patients in the treatment
group experienced symptom stabilization or even improvement during the clinical
period. When calculated as responder rate based on the percentage of patients
whose PSP Rating Scale scores improved or remained stable after six months of
treatment compared to baseline, 58.33% of PSP-RS type patients in the 0.56 mg
GV1001 group showed improvement or stabilization (see Figure3).
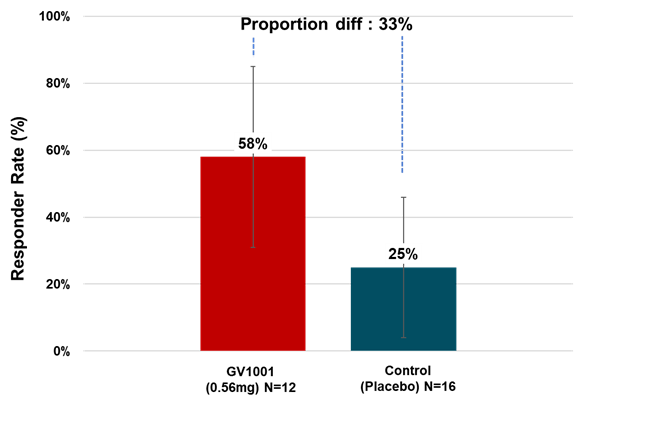
The safety profile of GV1001 in the Phase 2a
PSP Clinical Trial was consistent with prior safety data. GV1001 was generally
well-tolerated with no serious adverse events related to the drug reported.
Hyungsik Moon, CSO of GemVax, stated that
"this Phase 2a trial was an exploratory study to determine the optimal
dosage and find out how the peptide works on different subgroups. Although the
topline result did not achieve statistical significance, the evidence is strong
enough to consider moving forward to a pivotal trial and shows potential to
develop GV1001 as the world’s first treatment option for PSP.”
Experts at the Neuro2024 meeting welcomed the
results of the PSP trial as encouraging and expressed excitement for the drug
to enter a global Phase 3 clinical trial for further development.
“This pilot study was not fully powered and the
treatment duration with 6 months was short. Thus, statistically significant
confirmatory results could not be expected” said Peter Schüler, MD, Senior Vice
President of Drug Development at global CRO ICON. “Nonetheless, the observed
trends are very plausible and consistent in two domains: motor performance and
cognitive function, both favoring the lower dose group.”
“The trial identified the optimal dose, which
was one of the primary objectives of the Phase 2a study, and demonstrated
clinically meaningful benefits, namely full stabilization of the disease
compared to the placebo group,” said Dr. Schüler, adding “these topline results
provide a strong foundation for advancing to Phase 3.”
Dr. Günter U. Höglinger, Head of the Department
of Neurology, LMU Hospital, Munich, and a world-renowned expert in PSP,
commented: “very exciting Phase 2 level data with novel drug study with new
mechanisms of action. Data is preliminary but very promising and it is in line
with [GV1001] Alzheimer’s disease
clinical data. I look forward to further development and very excited to
participate and lead the [PSP] Phase 3 study.”
Dr. Kristophe Diaz, Director of CurePSP, said that
“we are encouraged by the results of the recent GemVax clinical trial, which
offer hope to the entire PSP community, including patients who currently have
no treatment options, their families and the physicians who care for them” and
that “we congratulate GemVax on the successful completion of this trial and
look forward to further developments that benefit the PSP community.” He also
said that “CurePSP remains committed to collaborating and supporting efforts
that bring hope and progress for those affected by this devastating disease.”
About Phase 2a PSP Clinical Trial (NCT05819658)
The Phase 2a PSP clinical trial was a 24-week, multicenter,
randomized, double-blind, placebo-controlled, prospective phase 2a exploratory
clinical trial to evaluate the safety and efficacy of GV1001 0.56 mg or 1.12 mg
compared to placebo for the treatment of patients with PSP. The primary outcome
of the study was change from baseline in the total score of PSP-Rating Scale
after 24 weeks of GV1001 administration. Secondary endpoints included change
from baseline in the total score of PSP-Rating Scale at 3 months, MoCA-K, K-FAB
and ES-ADL at both 3 and 6 months. Overall safety of GV1001 administration was also
assessed.
About GV1001
GV1001 is a synthetic peptide consisting of 16
amino acids based on the key sequence of telomerase. GV1001 has been studied
for the potential treatment of neurodegenerative diseases including Alzheimer’s
disease and PSP. In neurodegenerative diseases, GV1001 has been demonstrated to
modulate phenotypes of glial cells, and to regulate neuroinflammation. In
addition to the Phase 2a PSP clinical trial, a Phase 2 Alzheimer’s disease clinical
trial of GV1001 is currently ongoing in the U.S. and Europe (NCT05189210).
About PSP
Progressive supranuclear palsy is a rare progressive and adult-onset neurodegenerative disease that currently has no disease-modifying drug. Approximately seven in 100,000 people worldwide is affected by PSP and is more common in men. People over the age of 60 are mainly affected. The symptoms of PSP include loss of balance, changes in personality, weakness of eye movements, especially in the downward direction, difficulty in swallowing, slurred speech and cognitive impairment.
About GemVax & KAEL
GemVax & KAEL Co., Ltd. is a pioneering clinical-stage biopharmaceutical company based in Korea, dedicated to developing proprietary therapeutics for neurodegenerative diseases including progressive supranuclear palsy and Alzheimer’s disease. As for PSP, GemVax is currently conducting a Phase 2a study in Korea to evaluate the efficacy and safety of GV1001 in patients with PSP. Preparations are also underway for a global PSP clinical trial. In addition, GemVax is currently conducting a Phase 2 Alzheimer’s disease clinical trial in the U.S. and Europe. For more information, visit www.gemvax.com and follow us on Linkedin.
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995. Forward-looking statements may or may not include identifying words such as “plan,” “will,” “expect,” “anticipate,” “intend,” “believe,” “potential,” “continue,” and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties. All forward-looking statements are based on management’s current estimates, projections, and assumptions, and GemVax undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
댓글목록
등록된 댓글이 없습니다.
